Astra Zeneca clinical trial data review
Source
29 January 2021 EMA/94907/2021 Committee for Medicinal Products for Human Use (CHMP)
COVID-19 Vaccine AstraZeneca
Common name: COVID-19 Vaccine (ChAdOx1-S [recombinant])
Placebo
Brazil, UK: MenACWY (meningococcal group a, c, w-135, and y conjugate vaccine)
South Africa: Saline
Efficacy
Prevention, against hospitalization, against serious disease and death
Abbreviations: CI = Confidence Interval; ICU = Intensive Care Unit; NE = Not Evaluable; VE = Vaccine Efficacy. Source: Supplemental Tables IEMT 207.1, IEMT 207.2, IEMT 207.3, IEMT 207.4, IEMT 207.5.
COVID-19 events are adjudicated events based on virologically- confirmed results from RT-PCR or other nucleic acid amplification test. The 4 to 12 weeks dosing interval range corresponds to ≥ 28 days to ≤ 84 days.
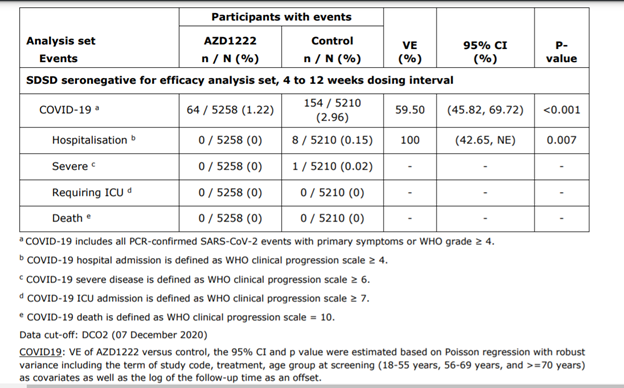
Occurring ≥15 Days Post Second Dose in the Pooled Analysis Set (COV002 + COV003), DCO2 (07
December 2020)
COVID19: VE of AZD1222 versus control, the 95% CI and p value were estimated based on Poisson regression with robust variance including the term of study code, treatment, age group at screening (18-55 years, 56-69 years, and >=70 years) as covariates as well as the log of the follow-up time as an offset.
Hospitalization: The maximum likelihood estimate of VE of AZD1222 versus control, the exact 95% CI (or 97.5% onesided) and p value were estimated based on stratified Poisson regression with Exact Conditional Method including treatment as factor, study code and age group at screening (18-55 years, 56-69 years, and >=70 years) as strata factors as well as the log of total number of participants for each combination of treatment and strata VE is defined as 1-(incidence from the AZD1222 arm / incidence from the control arm) expressed as a percentage, where the risk ratio is derived from Poisson regression with robust variance.
Efficacy against hospitalization
1-(hospitalization incidence from the AZD1222 arm / hospitalization incidence from the control arm) expressed as a percentage,
(1- (0/0.15)) = 100%
Vaccine Efficacy
The 95% CI for the VE is obtained by taking 1 minus the 95% CI of the risk ratio derived from the model. The observation period for the endpoint was 15 days post second dose up to 1 year in study.
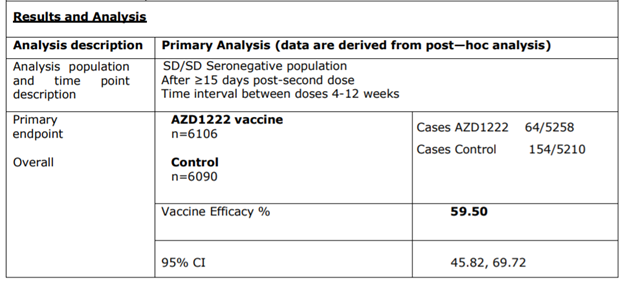
efficacy set, interval between doses 4-12 weeks)
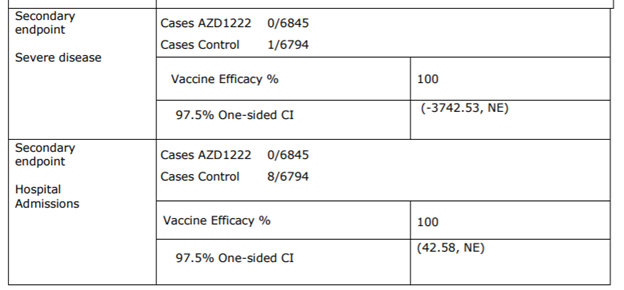
efficacy set, interval between doses 4-12 weeks)

Benefits
Preventing Covid-19
Base on positive on PCR test with primary symptoms (similar to a common cold) from incidences of 2,96 % (154⁄5210) in the placebo group versus 1.22% (64⁄5258 individuals) in the inoculated arm.
Preventing hospitalization
Based hospitalization incidence of 0.15 % (8⁄5210 individuals) in the placebo group versus o incidence in the inoculated arm
Preventing Severe disease
Cannot be assessed (Not applicable due to low incidence)
Preventing ICU
Cannot be assessed ( Not applicable due to low incidence)
Preventing death
Cannot ne assessed (Not applicable due to low incidence)
Safety
Risk assessment through incidences of adverse events.
Adversity
Adversity is calculated as 1-(AE incidence from the control arm / AE incidence from the AZD1222 arm) expressed as a percentage.
Incidence values: N values of the adverse event were used instead of actual incidence since the N value for the AZD122 arm is almost equal to the N values of the control arm.
We set the threshold of incidence to less than 0.1%: Adversity for Adverse event with incidence of less than 0.1% is insignificant.
| AZD1222 Inoculated N=12021 | Control N=11724 | Adversity % | |
| Participants with any investigational product Related Unsolicited AE | 3570 | 2172 | 39.16 |
| *System organ class uncoded* | 65 | 42 | 35.38 |
| Infections and infestations | 68 | 70 | –2.94 |
| Blood and lymphatic system disorders | 28 | 28 | 0.00 |
| Immune system disorders | 3 | 1 | 66.67 |
| Metabolism and nutrition disorders | 30 | 13 | 56.67 |
| Psychiatric disorders | 28 | 14 | 50.00 |
| Nervous system disorders | 1117 | 644 | 42.35 |
| Eye disorders | 32 | 19 | 40.63 |
| Ear and labyrinth disorders | 8 | 18 | –125.00 |
| Cardiac Disorders | 13 | 3 | 76.92 |
| Vascular disorders | 34 | 32 | 5.88 |
| Respiratory, thoracic, and mediastinal disorders | 128 | 121 | 5.47 |
| Gastrointestinal disorders | 323 | 184 | 43.03 |
| Skin and subcutaneous tissue disorders | 118 | 73 | 38.14 |
| Musculoskeletal and connective tissue disorders | 1081 | 448 | 58.56 |
| Renal and urinary disorders | 3 | 2 | 33.33 |
| Reproductive system and breast disorders | 5 | 4 | 20.00 |
| General disorders and administration site conditions | 2813 | 1505 | 46.50 |
| Investigations | 96 | 31 | 67.71 |
| Injury, poisoning and procedural complications | 11 | 10 | 9.09 |
Dose for Safety Analysis Set
The risks
Mostly adverse in terms of:
Cardiac Disorders (76.92%)
Musculoskeletal and connective tissue disorders (58.56%)
Metabolism and nutrition disorders (56.76%)
Psychiatric disorders (50%)
Uncertainties and limitations about unfavorable effects
Citations of main limitations
1
Long-term safety is considered a missing information in the Safety specification in the RMP, and will be characterised as part of the continuation of the pivotal clinical trial, other trials and a PASS.
2-
Safety data in participants with severe immunodeficiency, or participants with severe underlying disease
(including autoimmune or inflammatory disorders) are lacking, as all these populations were excluded from
the studies. The safety of AZD1222 in immunocompromised subjects will be evaluated post-authorization.
3-
Further, there is only very limited clinical experience in pregnant women, with 14 pregnant women in the
safety database who were exposed to AZD1222. Data from non-clinical studies do not indicate any harm
during pregnancy.* In the absence of clinical data to confirm lack of risks, risks during pregnancy remain,
albeit theoretical.
*there is no indication about the source of this affirmation; what non-clinical studies are they referring to?
Conclusion
It is questionable weather the benefit outweighs the risk since, to prevent SARS-CoV-2 infection with primary symptoms, like the common cold (100% efficacy) and possible hospitalization, one would take the risk of adversity in terms of cardiac disorders ( 76.92% ), musculoskeletal and connective tissue disorders (58.56% adversity ), metabolism and nutrition disorders (56.76% a) and psychiatric disorders (50%). Additionally, individuals with severe immunodeficiency, or with severe underlying disease (including autoimmune or inflammatory disorders) were excluded from the trial and a insignificant number of pregnant women in the database who were exposed to AZD1222. For these demographics, safety data are missing; safety and risks remain albeit theoretical. Overall, there is no telling what the future holds, these results being based on short-term clinical trial data.