Pharmacovigilance and VAERS
As you can well imagine the potential of things going wrong is enormous, short term as well as long term. This injected genetic material utilizes the cell resources to create the spike protein. Questions still remain: once produced by the cells, how is this spike protein eliminated from the cell? Meanwhile, the cell is depleted from its own resources…
Consequently, the possible visible clinical manifestations of cellular imbalance become so broad, ranging from blood clots, thrombocytopenia, Bell’ palsy, fever, opportunistic infections, sepsis, blindness, ear loss, or death that it makes it quite difficult to link to the “vaccination”. If the possible serious adverse events were limited to a mere four or five, the link (either from time of onset or pattern) would be much easier to make. Hence the great importance of pharmacovigilance and reporting of all adverse events.
Pharmacovigilance
It is clear that these “vaccines” are still being studied. So much remains unknown about this new technology; bio-distribution, pharmacokinetic, stability studies and long term effects to name a few. Truly, these are vaccine candidates that were rushed into the market. Therefore the vaccination campaign or public rollout are in fact vaccination trials, clinical trials to assess the long term safety of these vaccine candidates. They must be treated as such, with meaningful and thoughtful monitoring of every individual who participates in these trials. Of every arm that received those injections
As part of the conditions of their emergency use authorization of their vaccine candidate against SARS-Cov2, the manufacturing entities had to submit a pharmacovigilance plan to the regulatory agencies.
As an example, below is the pharmacovigilance plan from the Moderna for their experimental vaccine;
VAERS ( weekly update on pregnant women and Open VAERS)
Throughout the world various reporting systems are in place to capture these events, most are not mandatory and rely on the willingness of the individual towards reporting. In the US the VAERS reporting system is the main means of reporting.
VAERS: Excerpt from
https://www.historyofvaccines.org/content/articles/vaccine-development-testing-and-regulation
The CDC and FDA established The Vaccine Adverse Event Reporting System in 1990. The goal of VAERS, according to the CDC, is “to detect possible signals of adverse events associated with vaccines.” (A signal in this case is evidence of a possible adverse event that emerges in the data collected.) About 30,000 events are reported each year to VAERS. Between 10% and 15% of these reports describe serious medical events that result in hospitalization, life-threatening illness, disability, or death.
VAERS is a voluntary reporting system. Anyone, such as a parent, a health care provider, or friend of the patient, who suspects an association between a vaccination and an adverse event may report that event and information about it to VAERS. The CDC then investigates the event and tries to find out whether the adverse event was in fact caused by the vaccination.
The CDC states that they monitor VAERS data to
- Detect new, unusual, or rare vaccine adverse events
- Monitor increases in known adverse events
- Identify potential patient risk factors for particular types of adverse events
- Identify vaccine lots with increased numbers or types of reported adverse events
- Assess the safety of newly licensed vaccines
Not all adverse events reported to VAERS are in fact caused by a vaccination. The two occurrences may be related in time only. And, it is probable that not all adverse events resulting from vaccination are reported to VAERS. The CDC states that many adverse events such as swelling at the injection site are underreported. Serious adverse events, according to the CDC, “are probably more likely to be reported than minor ones, especially when they occur soon after vaccination, even if they may be coincidental and related to other causes.”
VAERS has successfully identified several rare adverse events related to vaccination. Among them are
- An intestinal problem after the first vaccine for rotavirus was introduced in 1999
- Neurologic and gastrointestinal diseases related to yellow fever vaccine
Additionally, according to Plotkin et al., VAERS identified a need for further investigation of MMR association with a blood clotting disorder, encephalopathy after MMR, and syncope after immunization (Plotkin SA et al. Vaccines, 5th ed. Philadelphia: Saunders, 2008).
The VAERS system is a passive reporting system since it is not mandatory to fill it out. In fact, evidence shows that the passive ways of reporting are quite ineffective in reporting serious adverse events or death.
‘’ In the past the passive surveillance system has been largely ineffective in reporting the serious suspected side effects of vaccinations, estimated to be between 2 and 10 percent in the UK and as little as 1% in the US.”
It should be a legal requirement for all suspected vaccine adverse reactions, not just for COVID vaccine related reactions, to be reported. Especially when they are manufactured via an Emergency Use authorization. Instead, we are observing that medical professionals community making their own on-the-spot judgement ( mostly that the reported situation is not related to the injection. A mandatory reporting would help to avoid the problems of under-reporting. Currently medical practices and organizations may well be conflicted by the financial entitlements paid to doctors and medics for vaccinating those registered with the practice/organization. This type of target based payment system may contribute to the high level of under-reporting .
All reported suspected adverse reactions should be routinely followed-up by the MHRA making contact with the patient’s doctor to ensure the individual has fully recovered or to assess the situation further. This could provide invaluable information and help to clarify which vaccines might cause harm and to whom, potentially avoiding future injuries and deaths.’’ ref Jab UK
As of may 2021, 4406 cases of deaths after vaccination against the Corona virus were reported through the US VAERS database. It is unsure if all those ultimate adverse events are currently under full investigation by the CDC.
Reading these reports on adverse events, one can observe the wide range of clinical manifestation, and quite unfortunately sometimes also, the reluctance of the health professionals to link the event to the “vaccination”. This, even with a very close time of onset from the time of vaccination. Since reporting these events is not mandatory, even in the case of Emergency Use Authorization, Conditional Marketing Authorizations, the serious adverse clinical manifestation will be underreported, underestimated. Therefore giving a false sense of safety, which is utterly detrimental to each individual who rely on the numbers the make their decision.
On a positive note, even with the great underreporting concern, some very important information can still be extracted using the VAERS database.
Pharmacovigilance on pregnant women
As previously mentioned, as part of the pharmacovigilance plan, pregnant woman are passively monitored after receiving receiving anti-covid injections.
To review the weekly updated VAERS on pregnant women
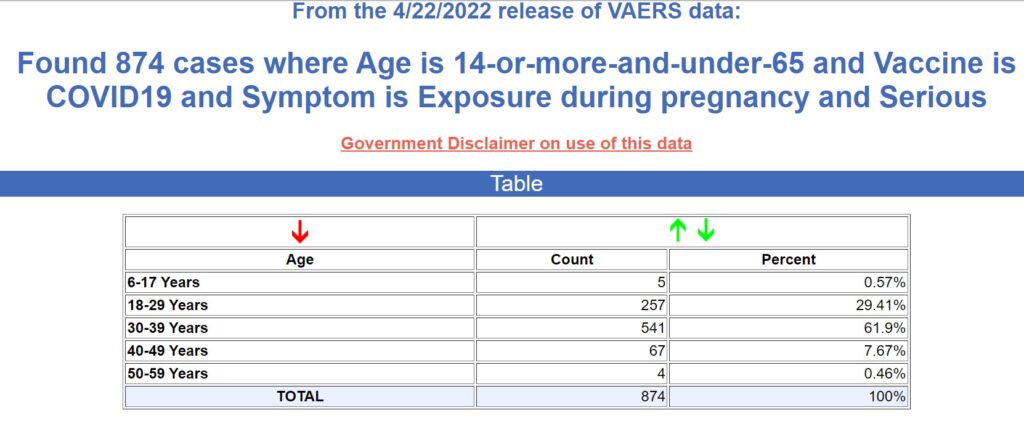
Open VAERS allows for different inquiries
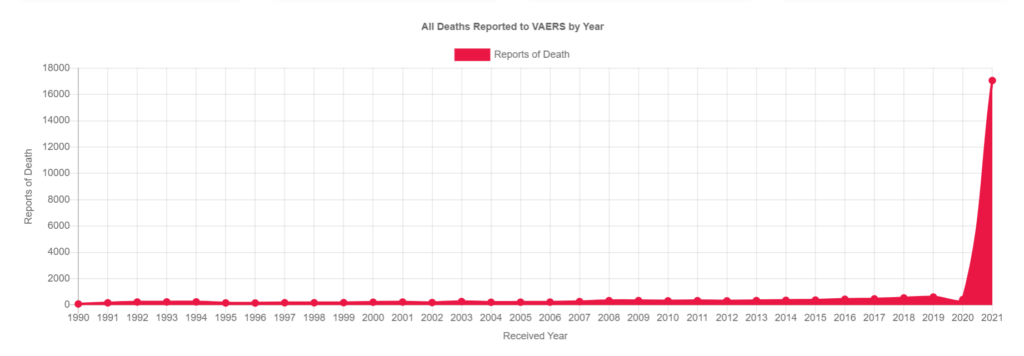
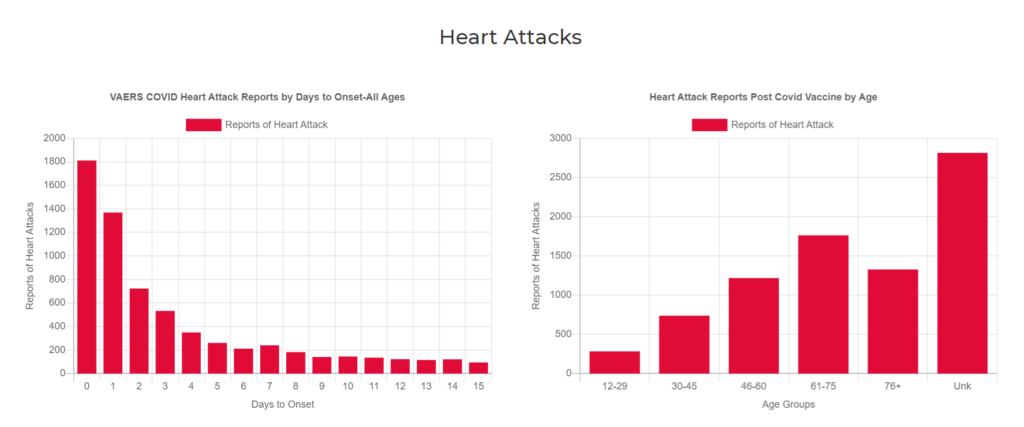
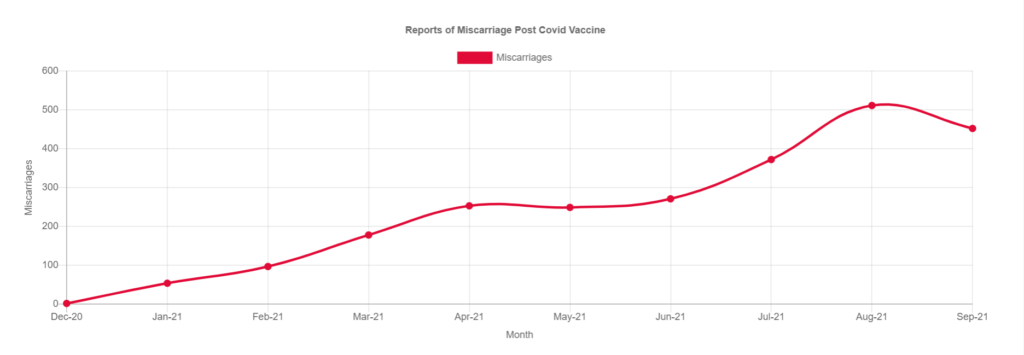
Share and donate
Share your experience family and friends from all your social platforms.
